Project management
First Think, Then Act?
… we believe in acting different.
We believe it is important to start; think big about the future and execute what you can do today.
Release your assumptions, focus on action and do it all with incredible speed.
This will help you move forward. Get things done. Make it all happen.
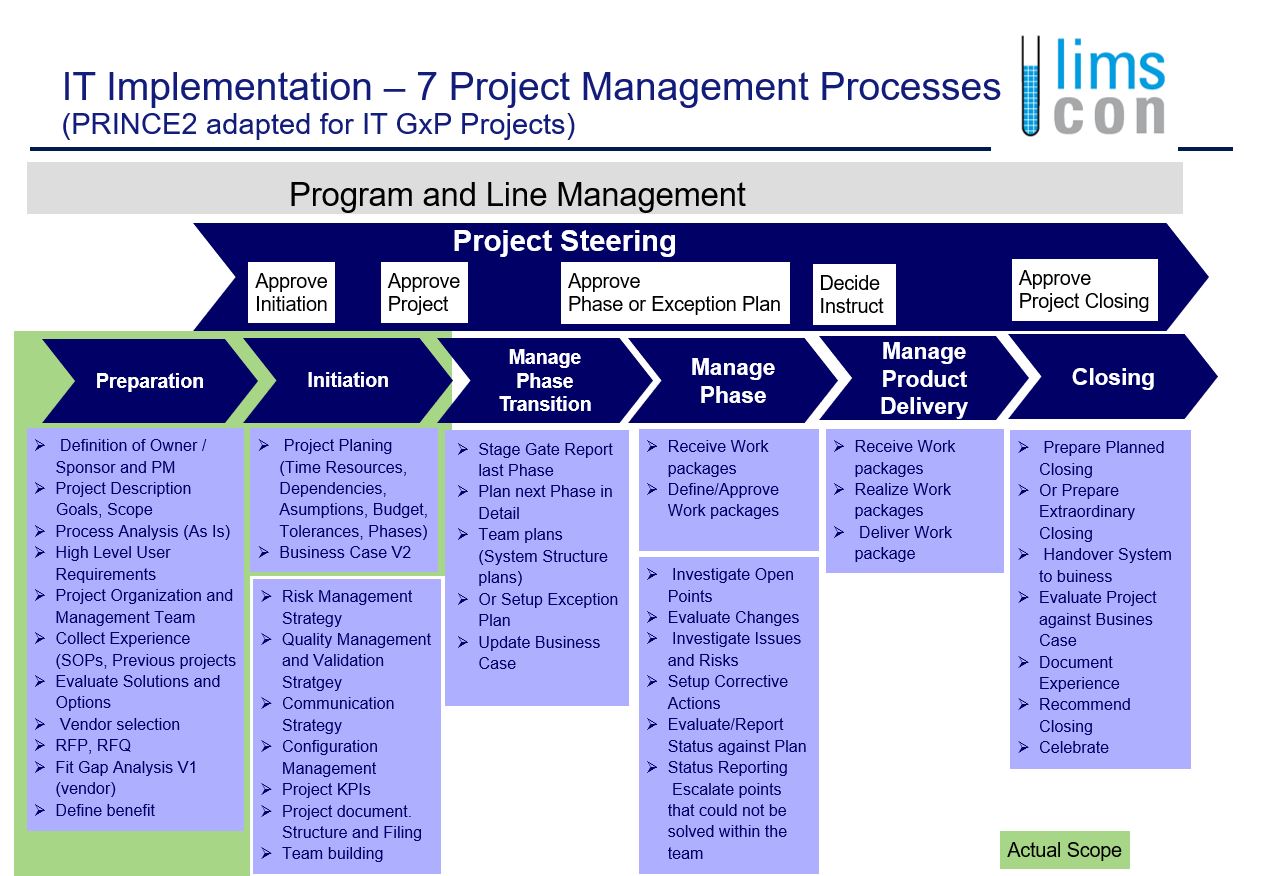
Based on above experience and with more then 40 years experience in implementation of LIMS systems we developed an own project methodology. The basis for this was the PRINCE2 model that has been adapted for the GxP regulated industry.
Requirements from authorities e.g. Pharm Eur, FDA, GxP, GAMP V are requesting a compliant Life Cycle Documentation in parallel to the technical implementation of systems.
Using these methods and tools it is possible to implement a fully validated LIMS, within a global organization of the Life Science Industry within 6 months.
THE KEY ELEMENTS OF OUR METHOD
- Business Case driven Project Controlling
- Project Phase model
- Product based planning
- Tool based Project planning (time, resources, budget)
- Weekly Status report and adjustment
- Change Management
- Issue and Risk Management
- Communication Strategy
- Continuos Resource Monitoring
- Tool based System validation (HPQuality SAP Testworkbench)
- Stake Holder Management
- Management of Expectations
- Team building
- System Validation
Are you interested?
Please contact us with any questions!